Nature Communications publication Van den Bossche Lab

Read the most recent publication from Jan Van den Bossche’s group on the role of macrophage ACLY during atherosclerosis in Nature Communications.
A popular summary (in Dutch) is published on the Amsterdam UMC news page.
Gijs Kooij

About
Gijs Kooij is a PI (assistant professor) at the Department of Molecular Cell Biology & Immunology as well as the MS center Amsterdam. His main research focus is to understand the natural process to resolve neuro-inflammation in order to provide new perspectives on the pathogenesis of chronic (unresolved) neuro-inflammatory diseases like multiple sclerosis (MS) and to reveal new treatment opportunities. He recently discovered endogenous lipid mediator circuits in the central nervous system (CNS) and revealed the absence of protective lipid mediators in various disease stages of MS. These findings in combination with personal Vidi (NWO) and Fellowship (Dutch MS society) grants have enabled him to establish his own research group on inflammation-resolution mechanisms in health and disease.
Research Line
(Re)solving MS: Understand and exploit endogenous protection mechanisms.
Inflammation is a host-protective response when properly orchestrated. Beside immune checkpoints, the inflammatory cascade boasts an additional checkpoint, driven by chemical lipid mediators that induce resolution of inflammation. These novel autacoids, called specialized pro-resolving lipid mediators (SPMs), not only inhibit the inflammatory response, they also actively terminate it, leading to the restoration of tissue homeostasis. Novel sensitive detection methods (i.e. lipidomics) have set the stage to identify and decode these SPMs, which opens new perspectives on the pathogenesis of chronic (unresolved) inflammatory diseases like multiple sclerosis (MS).
By using a state-of-the-art lipidomics and mass-spectrometry imaging approach in combination with primary cell cultures, ex vivo brain slice cultures, in vivo neuro-inflammation models and human post-mortem tissues, we will elucidate in the coming years how altered inflammation-resolving mechanisms underlie MS pathogenesis by investigating both fundamental (Vidi) and clinical (fellowship) aspects. His ultimate goal is to provide novel diagnostic, prognostic and therapeutic opportunities for MS and other chronic (neuro-)inflammatory diseases based on resolution pharmacology (Figure 1).
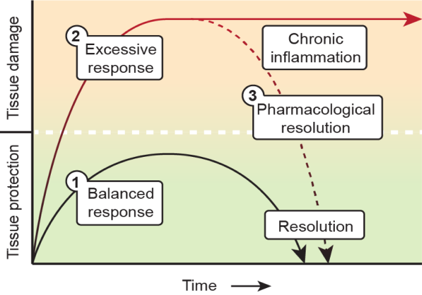
An effectively mounted inflammatory response will also trigger the activation of protective resolution pathways intended to safely terminate the inflammatory cascade and promote healing (1). Failed resolution can extend in time the actions of pro-inflammatory mechanisms resulting in prolonged (chronic) inflammation (2). I here propose that activation of endogenous circuits of resolution through novel resolution-based therapeutics (SPMs) can restore tissue structure and function to return to homeostasis (3).
Key publications
- Bogie J.F, Haidar M, Hendriks J.J.A and Kooij G. Fatty acid metabolism in the progression and resolution of CNS disorders. Advanced Drug Delivery Reviews. Conditionally accepted
- Derada Troletti C, Enzmann G, Chiurchiù V, Haghayegh N, Tietz S, Norris PC, van der Pol SMA, Serhan CN, de Vries HE, Engelhardt B and Kooij G. Pro-resolving lipid mediator Lipoxin A4 attenuates neuro-inflammation by modulating T cell responses and modifying the spinal cord lipidome. Cell Reports. Conditionally accepted.
- Kooij G, Derada Troletti C, Leuti A, Norris PC, Riley I, Albanese M, Ruggieri S, Libreros S, van der Pol SMA, van Het Hof B, Schell Y, Guerrera G, Buttari F, Mercuri NB, Centonze D, Gasperini C, Battistini L, de Vries HE, Serhan CN, Chiurchiù V. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica. 2019 Nov 28. pii: haematol.2019.219519. doi: 10.3324/haematol.2019.219519
- Kooij G, Kopplin K, Blasig R, Stuiver M, Koning N, Goverse G, van der Pol SM, van Het Hof B, Gollasch M, Drexhage JA, Reijerkerk A, Meij IC, Mebius R, Willnow TE, Müller D, Blasig IE, de Vries HE. Disturbed function of the blood-cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathol. 2014 Aug 128(2): 267-77
- Kooij G, Kroon J, Paul D, Reijerkerk A, Geerts D, van der Pol SM, van Het Hof B, Drexhage JA, van Vliet SJ, Hekking LH, van Buul JD, Pachter JS, de Vries HE. P-glycoprotein regulates trafficking of CD8+ T cells to the brain parenchyma. Acta Neuropathol. 2014 May;127(5): 699-7112Aleyd E, van Hout MW, Ganzevles SH, Hoeben KA, Everts V, Bakema JE, van Egmond M. IgA enhances NETosis and release of neutrophil extracellular traps by polymorphonuclear cells via Fcα receptor I. J Immunol. 2014 Mar 1;192(5):2374-83.
Group members

Jelle Broos, MSc
PhD student
The primary focus of my research is the involvement of bioactive lipid mediators in Multiple Sclerosis. We are currently screening several large MS cohorts for these mediators and try to link their levels to clinical and MRI data in order to gain more insights in their function.

Julia Konings, MSc
PhD student
In my research we aim to explore the role of resolution in the development and pathogenesis of MS, focusing on the underlying mechanisms of impaired specialized pro-resolving lipid mediator production as observed in MS. This will be done by investigating expression levels and epigenetic signatures of receptors, transporters and enzymes involved in their biosynthesis and degradation.

Marc Franssen
PhD student
I focus mainly on lipid mediators in stroke and subarachnoid hemorrhage. In doing so I hope to explore how these lipid mediators modulate these pathologies. Specifically, the immunological response and that of the cells that make up the NVU is of great interest to me. For that purpose I am currently using LC-MS/MS, as well as trying to set a up a in vitro stroke model and hope to make use of 2-photon microscopy in the future.

Wing Hee Fung, MD
PhD student
My research interests center around the neurovascular crosstalk in health and disease, especially in Multiple Sclerosis. In addition, my research focuses on immunomodulatory lipids to promote neurovascular health. High-throughput analysis is used to identify biomarkers for prediction of disease state, disease progression and therapeutic response in Multiple Sclerosis.

Wing Ka Fung
research technician
My main research focus is to unravel the pathogenesis of Multiple Sclerosis by performing different molecular techniques, cell culture and immunohistochemistry.
Other PI's
Sue Gibbs
Febe van Maldegem
Elga de Vries
Sandra van Vliet
Reina Mebius
Yvette van Kooyk
Jack van Horssen
Joke den Haan
Juan J. Garcia Vallejo
Marjolein van Egmond
Elga de Vries

Research Line
Neuro-immunology: The neurovascular unit in health and disease
Altered activation of the immune system, neuroinflammation (marked by activated astrocytes and microglia), and dysfunction of the neuro-protective brain barriers are pathological hallmarks of many neurodegenerative disorders, such as multiple sclerosis (MS), various forms of dementia, including Alzheimer’s disease (AD), and stroke. It becomes increasingly clear that chronic neuroinflammation, an altered immune response and neurovascular dysfunction may even be causal for onset and progression of such cognitive disorders, but underlying mechanisms remain unknown. The translational research of the De Vries group is therefore focused on understanding how alterations at the level of the immune system and the brain barriers underlie neuroinflammatory and neurodegenerative conditions. In the current research, we aim to define underlying pathways that initiate neuro-inflammation as potential targets for treatment and identify if such alterations may serve as biomarkers for disease in well-defined patient cohorts. A better understanding of such pathological processes may in future not only lead to new diagnostic tools that reflect ongoing neuro-inflammation and brain barrier dysfunction, but may also lead to potential novel intervention strategies to fight neurogical disorders.
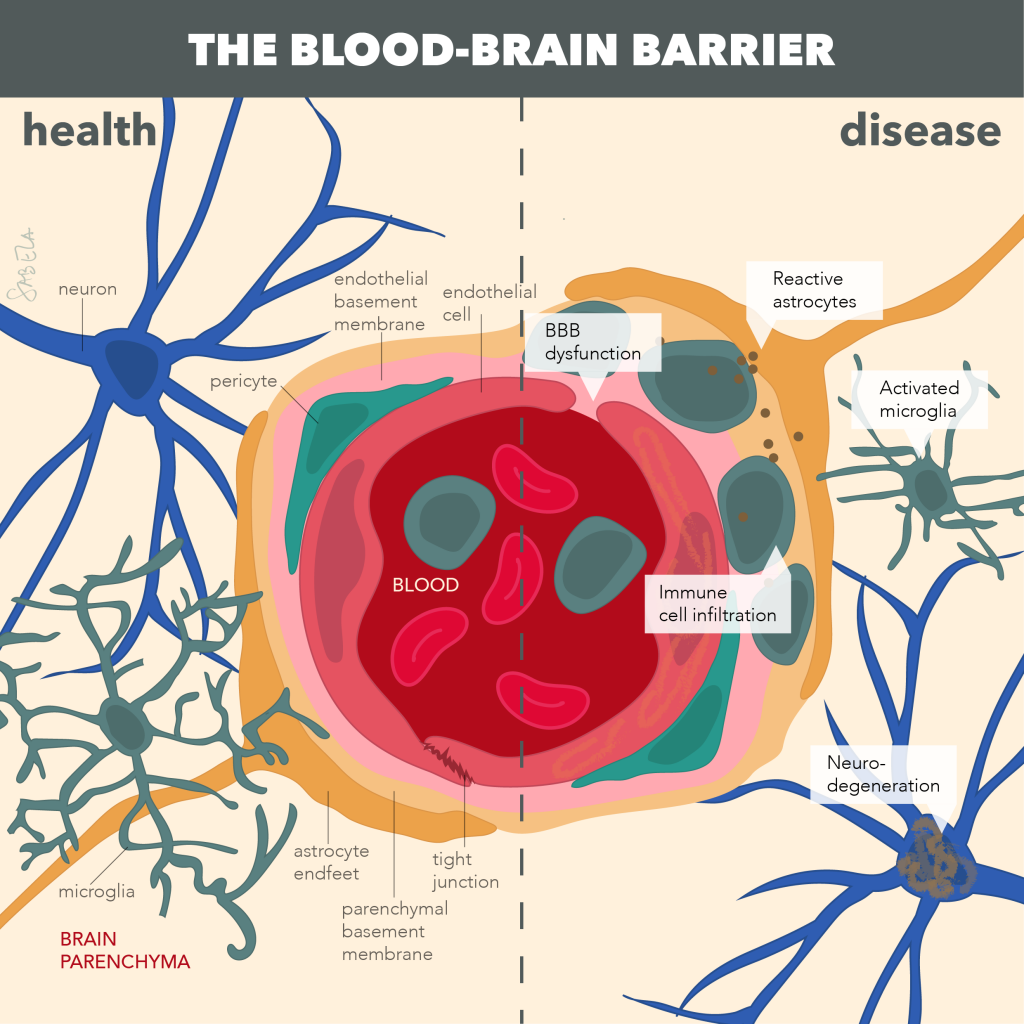
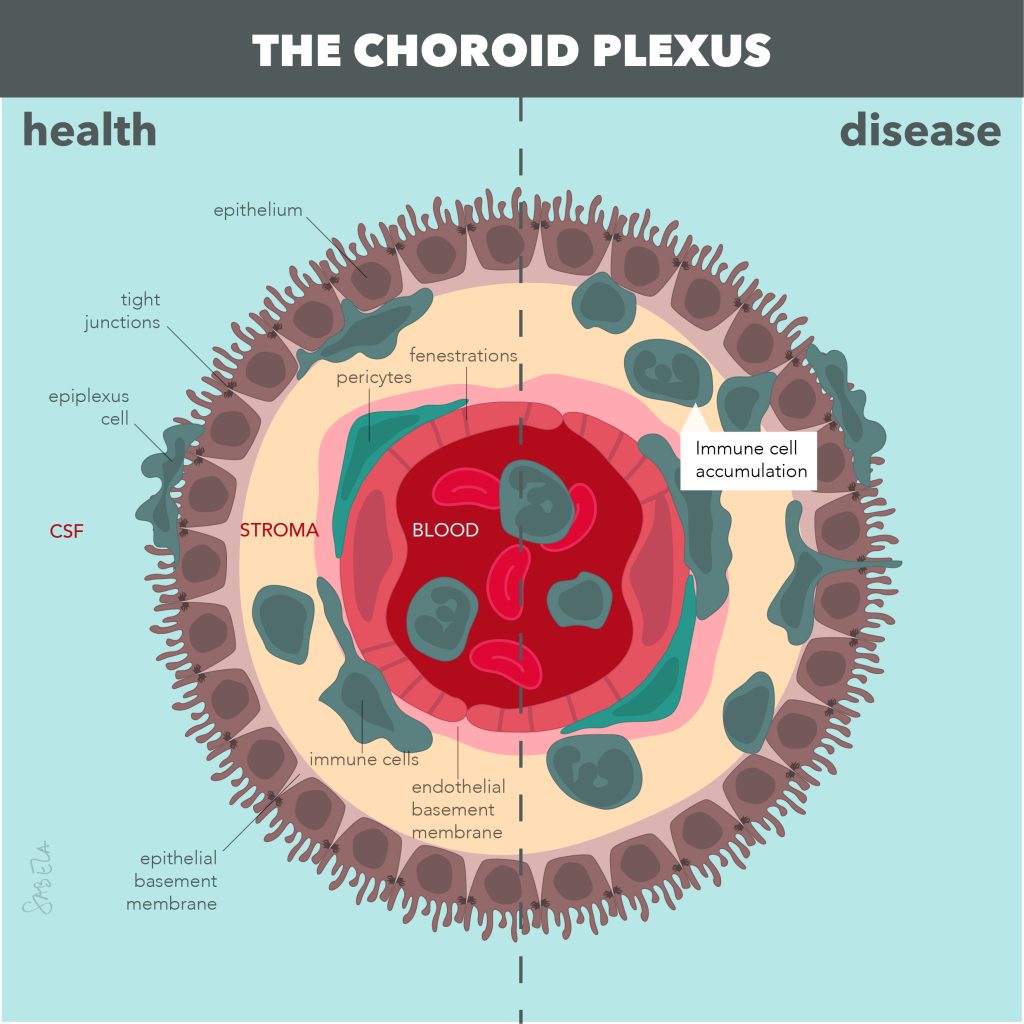
Key publications
- van Olst L, Coenen L, Nieuwland JM, Rodriguez-Mogeda C, de Wit NM, Kamermans A, Middeldorp J, de Vries HE. Crossing borders in Alzheimer’s disease: A T cell’s perspective. Adv Drug Deliv Rev. 2022 Sep;188:114398. doi: 10.1016/j.addr.2022.114398. Epub 2022 Jun 30.PMID: 35780907
- Rodríguez-Lorenzo S, van Olst L, Rodriguez-Mogeda C, Kamermans A, van der Pol SMA, Rodríguez E, Kooij G, de Vries HE. Single-cell profiling reveals periventricular CD56brightNK cell accumulation in multiple sclerosis. 2022 May 10;11:e73849. doi: 10.7554/eLife.73849.PMID: 35536009
- Wevers NR, Nair AL, Fowke TM, Pontier M, Kasi DG, Spijkers XM, Hallard C, Rabussier G, van Vught R, Vulto P, de Vries HE, Lanz HL. Modeling ischemic stroke in a triculture neurovascular unit on-a-chip. Fluids Barriers CNS. 2021 Dec 14;18(1):59. doi: 10.1186/s12987-021-00294-9.PMID: 34906183
- Rodríguez-Lorenzo S, Ferreira Francisco DM, Vos R, van Het Hof B, Rijnsburger M, Schroten H, Ishikawa H, Beaino W, Bruggmann R, Kooij G, de Vries HE. Altered secretory and neuroprotective function of the choroid plexus in progressive multiple sclerosis.Acta Neuropathol Commun. 2020 Mar 19;8(1):35. doi: 10.1186/s40478-020-00903-y.PMID: 32192527
- Wouters E, de Wit NM, Vanmol J, van der Pol SMA, van Het Hof B, Sommer D, Loix M, Geerts D, Gustafsson JA, Steffensen KR, Vanmierlo T, Bogie JFJ, Hendriks JJA, de Vries HE. Liver X Receptor Alpha Is Important in Maintaining Blood-Brain Barrier Function.Front Immunol. 2019 Jul 31;10:1811. doi: 10.3389/fimmu.2019.01811. eCollection 2019.PMID: 31417573
Group members

Carla Rodriguez Mogeda, MSc
PhD student
My research focuses on understanding meningeal inflammation in progressive multiple sclerosis. I am specifically interested in the role of B cells, in how they get to the meninges and how they might interact, directly or indirectly, with other immune cells such as microglia. To do this, I use different single-cell techniques for proteomics and transcriptomics, and confocal and multispectral microscopy..

Chaja van Ansenwoude, MSc
PhD student
My research is focused on the role of NK cells in MS pathogenesis, specifically CD56bright NK cells. This cell population appears to accumulate in the brain of MS patients and exhibits an immunomodulatory and migratory phenotype, suggesting a protective role for these cells in MS. We aim to further unravel the mechanisms underlying CD56bright NK cell effector function by studying cellular interactions, immunoregulatory and neuroprotective capacities, mechanisms of migration, and correlations with clinical markers. For this, I will make use of multispectral microscopy, single cell mass cytometry, in vitro BBB and BCSFB models, and co-culture experiments.

Hannah van der Stok, MSc
Research technician
My work focuses on blood brain barrier functioning. I am specifically interested in a protein named FHL2, which potentially plays a role in blood brain barrier dysfunction. Intracellular mechanisms involved in this process are investigated using in vitro models with lentiviral modifications and various molecular assays.

Henrique Nogueira Pinto, MSc
PhD student
My research is centered on the development of a brain-on-a-chip, combining iPSC-derived cerebral organoids and a bioengineered blood-brain barrier (BBB). This platform will be used to assess the capability of certain compounds to cross the BBB and their neurobiological effects, either in a healthy or disease-specific context.

Inge Mulder, PhD
Senior postdoctoral researcher
My research is focused on the inaccurate reperfusion of the microvasculature of the brain, after recanalization therapy (thrombolysis or thrombectomy) in acute ischemic stroke.
I aim to unravel underlying pathophysiological mechanisms of this phenomenon in order to find new therapeutic strategies to improve treatment efficacy. To do this, I use a translational approach including pre-clinical in-vivo 2-photon microscopy, MRI and Mass spectrometry as well as clinical data.

Merel Rijnsburger, PhD
Post-doctoral researcher
Studying the role of hormones in MS pathophysiology

Nienke de Wit, PhD
Postdoctoral researcher
My research focuses on the blood-brain barrier in health and disease on a
molecular level. Using in vitro cell systems and post-mortem human brain
tissue I try to understand specific pathways that are necessary for maintaining
proper barrier function.

Parand Zarekiani, MSc
PhD student
My research focuses on leukodystrophies, which are genetic disorders that primarily affect the brain white matter. More specifically, I study how the neurovascular unit is involved in these diseases. In the past years, the neurovascular unit has been overlooked in leukodystrophies, while it can have significant implications in the disease progression and in treatments. Using a multidisciplinary approach, I collaborate with pathologists, clinicians and biologists in order to unravel the underlying mechanisms in these diseases. For these studies, I use human post-mortem tissue, in vivo and in vitro approaches.

Ruud Fontijn, PhD
Researcher
My work focuses on the blood-brain barrier, in particular on the endothelial component thereof. Mechanisms of blood-brain barrier failure in neurodegenerative diseases are assessed using in vitro models, impedance measurements, lentiviral genetic modification and various biochemical and molecular biological techniques.

Susanne van der Pol, Ing
Research Technician
Expertise in cell isolations, iPSC, cell-based assays, immunostainings (IHC, ICC, IF), CyTOF, FACS.
Other PI's
Sue Gibbs
Febe van Maldegem
Gijs Kooij
Sandra van Vliet
Reina Mebius
Yvette van Kooyk
Jack van Horssen
Joke den Haan
Juan J. Garcia Vallejo
Marjolein van Egmond
Sandra van Vliet

About
During her cum laude PhD and postdoctoral years Sandra J. van Vliet studied the impact of glycosylation and glycan-binding receptors on dendritic cell biology and specialized in the field of glyco-immunology. She obtained a prestigious NWO-VENI grant in 2010 that allowed her to dissect the signaling properties of glycan-binding receptors. With grants from the Dutch Cancer Society, Cancer Center Amsterdam and European Marie Curie ITN network she has established her own independent research group that aims to unravel how tumor glycans affect tumor progression, metastasis and anti-tumor immunity. In 2017, Sandra van Vliet was awarded the female career award ASPASIA from the Netherlands Organisation for Scientific Research (NWO). With the ASPASIA she established the Amsterdam UMC Women in Science Fund, which aims to accelerate the careers of young female scientists by providing grants for international work visits and networking.
Research Lines
- Impact of tumor cell glycosylation on tumor cell biology and anti-tumor immunity
Cellular glycosylation is a highly dynamic process that alters upon activation, inflammation, and oncogenic transformation. Although it has been known for decades that cell surface glycans are highly diverse, it is still unclear how glycan heterogeneity is established and how this impacts tumor cell biology and tumor-immune cell recognition. We employ CRISPR/Cas9 genome engineering (both gene knockout as well as gene induction strategies) to generate isogenic tumor glycovariant cell lines and organoids. We assess the effect of tumor cell glycosylation on 3D tumor growth as well as tumor cell differentiation and immune cell interactions, mainly in the context of colorectal cancer. We address how the tumor glyco-code affects anti-tumor immunity as well as the response to immunotherapy. - Molecular recognition and signaling of lectin receptors within the immune system
Within the immune system, glycan blue prints are decoded by specific glycan-receptors, such as the C-type lectin (CLRs) or the Siglec receptors. These glycan-binding receptors are crucial in regulating the balance between immunity and tolerance and several CLRs and Siglecs seem to endow a tolerogenic phenotype on antigen presenting cells (APCs) to dampen potential immune responses directed against the tumor. We study on a molecular level how different glycan ligands affect lectin signaling and subsequent reprogramming of the APCs. In addition, we design targeting moieties to harness lectin function in order to to adjust and fine-tune immune responses.
How does the tumor-associated glycome affect anti-tumor immunity?
Within the immune system, glycan blue prints are decoded by specific glycan-receptors, such as the C-type lectin (CLRs) or the Siglec receptors. These glycan-binding receptors are crucial in regulating the balance between immunity and tolerance and several CLRs and Siglecs seem to endow a tolerogenic phenotype on antigen presenting cells (APCs) to dampen potential immune responses directed against the tumor. We study this by correlating lectin binding to disease progression and immune cell infiltration and activation in human tumor samples.
To assess the immunological consequences of altered tumor glycosylation on a molecular level, we stimulate lectin receptors on dendritic cells and macrophages with synthetic glycoconjugates and glycan-modified dendrimers. These glycan-conditioned APC are subsequently profiled by RNAseq and characterized for their maturation marker expression, cytokine secretion and their ability to instruct effector and regulatory T cell responses. We are currently addressing whether targeting of lectin receptors also alters the metabolism of antigen presenting cells.
Key publications
- van der Meijs NL, Travecedo MA, Marcelo F, van Vliet SJ. The pleiotropic CLEC10A: implications for harnessing this receptor in the tumor microenvironment. Expert Opin Ther Targets. 2024 Jul 3:1-12. doi: 10.1080/14728222.2024.2374743.
- van der Haar Àvila I, Zhang T, Lorrain V, de Bruin F, Spreij T, Nakayama H, Iwabuchi K, García-Vallejo JJ, Wuhrer M, van Kooyk Y, van Vliet SJ. Limited impact of cancer-derived gangliosides on anti-tumor immunity in colorectal cancer. Glycobiology. 2024 May 26;34(7):cwae036. doi: 10.1093/glycob/cwae036.
- Gabba A, Attariya R, Behren S, Pett C, van der Horst JC, Yurugi H, Yu J, Urschbach M, Sabin J, Birrane G, Schmitt E, van Vliet SJ*, Besenius P*, Westerlind U*, Murphy PV*. MUC1 Glycopeptide Vaccine Modified with a GalNAc Glycocluster Targets the Macrophage Galactose C-type Lectin on Dendritic Cells to Elicit an Improved Humoral Response. J Am Chem Soc. 2023 Jun 21;145(24):13027-13037. doi: 10.1021/jacs.2c12843. *shared last author
- Cornelissen LAM, Blanas A, van der Horst JC, Kruijssen L, Zaal A, O’Toole T, Wiercx L, van Kooyk Y, van Vliet SJ. Disruption of sialic acid metabolism drives tumor growth by augmenting CD8(+) T cell apoptosis. Int J Cancer. 144(9):2290-2302 (2019)
- Marcelo F, Supekar N, Corzana F, van der Horst JC, Vuist IM, Live D, Boons GPH, Smith DF, van Vliet SJ. Identification of a secondary binding site in human macrophage galactose-type lectin by microarray studies: Implications for the molecular recognition of its ligands. J Biol Chem. 294(4):1300-1311 (2019).
- Blanas A, Cornelissen LAM, Kotsias M, van der Horst JC, van de Vrugt HJ, Kalay H, Spencer DIR, Kozak RP, van Vliet SJ. Transcriptional activation of fucosyltransferase (FUT) genes using the CRISPR-dCas9-VPR technology reveals potent N-glycome alterations in colorectal cancer cells. Glycobiology. 29(2):137-150 (2019)
- Blanas A, Sahasrabudhe NM, Rodríguez E, van Kooyk Y and van Vliet SJ. Fucosylated antigens in cancer: an alliance towards tumor progression, metastasis and resistance to chemotherapy. Front Oncol. 8:39 (2018)
This article was selected for a special edition for World Cancer day 2019, highlighting a selection of top articles published in Frontiers in Oncology over the prior 12 months. - Lenos K, Goos JACM, Vuist IM, den Uil SH, Delis-van Diemen PM, Belt EJTh, Stockmann HBAC, Bril H, de Wit M, Carvalho B, Giblett S, Pritchard CA, Meijer GA, van Kooyk Y, Fijneman RJA, van Vliet SJ. MGL ligand expression is correlated to BRAF mutation and associated with poor survival of stage III colon cancer patients Oncotarget 6(28):26278-90 (2015) ‒ IF 5.2, corresponding author


Group members

Remi Hatinguais, MSc
PhD student

Irene van der Haar Àvila, MSc
PhD student
The focus of my PhD project is to evaluate the impact of sialylation on the anti-tumor response in colorectal cancer (CRC) by generating CRISPR/Cas9-based CRC cell lines with different levels of sialic acids. We aim to identify specific sialo-signatures that help in predicting response and success of cancer immunotherapy. For this, we make use of in vivo tumor models, multicolor (spectral) flow cytometry, microscopy imaging, IHC, co-culture assays, and RNA sequencing data.

Nadia van der Meijs, MSc
PhD student
I’m investigating the signaling of the C-type lectin receptor MGL in dendritic cells in more detail by looking at, for example, the cytokine production, co-stimulatory and inhibitory receptor expression and gene expression patterns. With different MGL ligands, that induce a different conformation in MGL after binding, we hope to steer the immune response started by the dendritic cells in different directions.

Victor Lorrain, MSc
Research technician
Investigating the impact of tumor glycosylation on anti-tumor immunity
Other PI's
Sue Gibbs
Febe van Maldegem
Gijs Kooij
Elga de Vries
Reina Mebius
Yvette van Kooyk
Jack van Horssen
Joke den Haan
Juan J. Garcia Vallejo
Marjolein van Egmond
Reina Mebius

About
Reina Mebius is professor in Molecular Cell Biology at the Department of Molecular Cell Biology & Immunology. She has contributed to the field of lymphoid organ development showing that communication between stromal cells and immune cells is crucial for the forming of lymph nodes. The realization that immune cell interaction with stromal cells is central to lymph node formation started another new research field in which the importance of stromal cells for the immune system is studied. Now the different roles that various stromal cell subsets have on the immune response during homeostasis and disease is further dissected using advanced techniques such as single cell RNA sequencing and fluorescence is situ hybridization, in order to discover novel points of interference for targeted therapy in autoimmunity and cancer.
Research Line
Functional development of the immune system
Lymphoid organs are built by a network of stromal cells, which are instrumental for formation of these organs in early ontogeny. Secondary lymphoid organs and their compartments greatly enhance the odds that rare antigen-specific T cells encounter dendritic cells that present the antigen and are thus crucial for effective immune responses. We study this process of lymph node formation and the cellular and molecular mechanisms that are required to form these highly organized lymphoid organs. Special attention is paid to the differentiation requirements of stromal precursor cells to the different stromal subsets that are present in adult lymphoid organs.
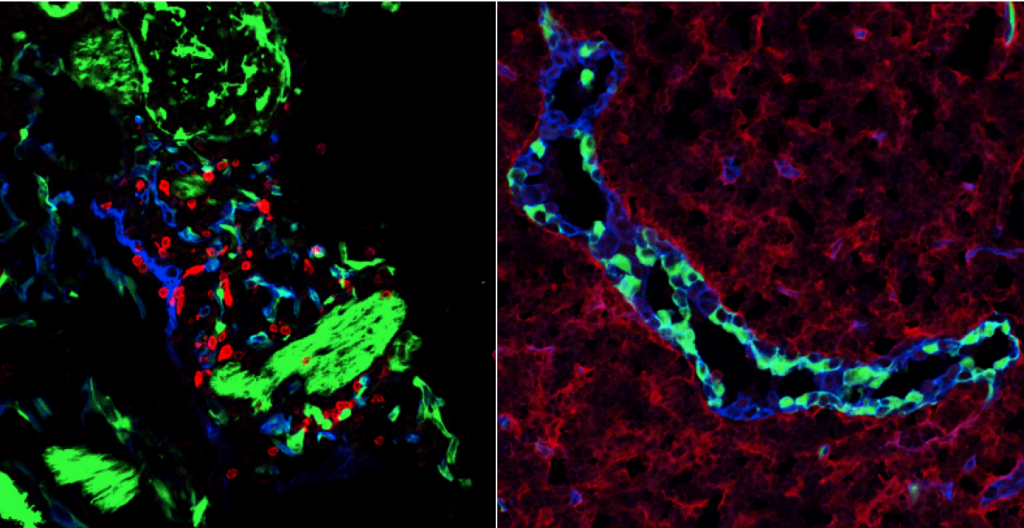
Environmental control of the immune response
Stromal cells within the lymph nodes are able to convey location specific as well as survival signals to immune cells. The formation of lymphoid compartments is not restricted to ontogeny, since ectopic lymphoid structures are formed during chronic inflammatory diseases as well in solid tumours, where they can either contribute to the inflammatory response or an anti-tumour response, respectively. Within the organized structures various different stromal subsets are present, each with their own function. Further characterization of these functions reveal more specifics by which the immune system is controlled by its environment.
In addition, the micro environmental control outside of organized lymphoid organs are studied in inflammatory diseases as well as in cancer in order to better understand how the micro-environment contributes to disease.
Recent key publications
1. Gago da Graça C, van Baarsen LGM, Mebius RE. Tertiary Lymphoid Structures: Diversity in Their Development, Composition, and Role. J Immunol. 2021 Jan 15;206(2):273-281.
2. Nadafi R, Gago de Graça C, Keuning ED, Koning JJ, de Kivit S, Konijn T, Henri S, Borst J, Reijmers RM, van Baarsen LGM, Mebius RE. Lymph Node Stromal Cells Generate Antigen-Specific Regulatory T Cells and Control Autoreactive T and B Cell Responses. Cell Rep. 2020 Mar 24;30(12):4110-4123.
3. Bar-Ephraim YE, Koning JJ, Burniol Ruiz E, Konijn T, Mourits VP, Lakeman KA, Boon L, Bögels M, van Maanen JP, Den Haan JMM, van Egmond M, Bouma G, Reijmers RM, Mebius RE. CD62L Is a Functional and Phenotypic Marker for Circulating Innate Lymphoid Cell Precursors. J Immunol. 2019 Jan 1;202(1):171-182.
4. Bar-Ephraim YE*, Cornelissen F*, Papazian N, Konijn T, Hoogenboezem RM, Sanders MA, Westerman BA, Gönültas M, Kwekkeboom J, Den Haan JMM, Reijmers RM, Mebius RE*, Cupedo T*. 2017. Cross-Tissue Transcriptomic Analysis of Human Secondary Lymphoid Organ-Residing ILC3s Reveals a Quiescent State in the Absence of Inflammation. Cell Rep. 21(3):823-833. * Equal contribution.
5. Koning JJ, Konijn T, Lakeman KA, O’Toole T, Kenswil KJ, Raaijmakers MH, Michurina TV, Enikolopov G, Mebius RE. 2016. Nestin-Expressing Precursors Give Rise to Both Endothelial as well as Nonendothelial Lymph Node Stromal Cells. J Immunol. 197:2686-94.
Group members

Andrew Morrison, MSc
PhD student
My project aims to develop a functional 3D organotypic lymph node with integrated lymphatics that mimic adaptive immune responses. Combining research on lymph node stromal cells and current in-house expertise of tissue engineering & microfluidic devices, the overall objective of the project is to design an organ-on-chip model that features lymphatic drainage of an immune competent gut into a lymph node. The immunosurveillance capability of the model will then be assessed by inducing an inflammatory response, leading to its availability as a future platform for rheumatoid arthritis drug testing.

Daphne Panocha, MSc
PhD student
My work focuses on the development of a human immunocompetent 3D lymph node-on-chip model with integrated lymphatics that mimics adaptive immune responses. We aim to incorporate this 3D lymph node in an organ-on-chip platform by combining it with an immunocompetent skin-on-chip model in a lymph vascularized chip. Functionality of this skin-and lymph node-on-chip model will be assessed by the topical application of contact sensitizers. Important aspects of my project include lymph node stromal cell culture and tissue engineering.

Eelco Keuning, MSc
Lead technician
As lead technician my task is to organize and manage the laboratories at our department so everything runs smoothly. I do this together with our lab manager Ed Döpp. In this management position my role is to focus on quality and safety. In addition I’m the experimental animal research coordinator of the department, for which I function as central contact to all involved parties and can support in many different in vivo models, biotechnical techniques as well as several operations.

Estefany Burniol Ruiz, MD
PhD student
In my research I focus on ILCs in IBD and the changing of homing markers. In 2019 my project became a part of the TIMID consortium. With TIMID we will be able to look at immune-cell alterations in PBMC’s in various immune-mediated diseases. Within the consortium we will be able to combine this with the data from all the other specialists in order to get better insights in these diseases.

Janna Roet, MSc
PhD student
The focus of my project is to unravel the mechanisms of self-antigen expression in lymph node stromal cells, which is needed for the induction of peripheral tolerance. By using a whole-genome CRISPR activation screen we aim to identify the regulatory factors of self-antigen expression. Discovering these regulators will give more insights in fundamental mechanisms of autoimmunity and might be used to design new therapeutic strategies. For this project I make use of flow cytometry, multiplex microscopy, vector cloning and transfections, transductions and RNA sequencing data.

Lotte de Winde, PhD
Research associate
I study the tumor-promoting role of lymph node stromal cells in B-cell lymphoma, and in sentinel lymph nodes draining from solid tumors (melanoma, breast cancer). More specifically, I investigate how interactions between lymph node stromal cells and tumor cells contribute to tumor progression by driving tumor cell dissemination and/or escaping anti-tumor immunity. In my projects, I use primary human lymph node and tumor material as well as cell lines in 2D and 3D co-culture assays, multicolor (spectral) flow cytometry, (advanced) fluorescence microscopy, and molecular and cell biology assays

Mariateresa Coppola, MD, PhD
Postdoctoral researcher
Unrevealing new facets of the human immune responses fascinates me enormously, especially since I believe this knowledge can be translated into a better prevention and treatment of disabling immune-mediated diseases.
Within the TargetToB consortium (Target to B – Target to B (target-to-b.nl)), I want to deep-phenotype B and follicular T-helper cells by mass-cytometry and further characterize newly-discovered cell populations with functional assays. This approach will increase our comprehension of the heterogeneous cell subsets involved in the pathogenesis of auto-immune diseases and cancer which is indispensable to identify novel biomarkers predicting therapy-outcomes and disease-prognosis.

Michael de Kok, MSc
Embedded / Supporting Bioinformatician
My function is to support the entire department with all bioinformatics needs. With so many different projects and data acquisition methods within the department, there is a need for a dedicated person to develop, maintain and teach different scripts and pipelines for all data analyses. This can be transcriptomics, proteomics, imaging or any other conceivable pipeline. People can approach me for assistance with their analyses and quickly get their current problems solved. In addition I manage data, e-lab journals and co-host multiple meetings and courses concerning bioinformatics as well as teaching subjects such as programming and good data practices.

Tanja Konijn, Ing
Research technician
I work together with Postdocs and AIO’s on several projects concerning lymph development and mucosal immunology. Since 2014 I became, in combination with my function as technician, an operator at the Microscopy & Cytometry Core Facility (MCCF).
Other PI's
Sue Gibbs
Febe van Maldegem
Gijs Kooij
Elga de Vries
Sandra van Vliet
Yvette van Kooyk
Jack van Horssen
Joke den Haan
Juan J. Garcia Vallejo
Marjolein van Egmond
Yvette van Kooyk

About
Yvette van Kooyk is professor in Molecular Cell Biology and Immunology, head of the department Molecular Cell Biology and Immunology and PI of the Dendritic Cell Immunobiology group. Van Kooyk’s research team studies innate and adaptive immune responses guided by glycosylation. Her team unravels cellular communication driven by modified glycoproteins/lipids in cancer, allergy and autoimmunity. Central in her work is the development of new glycan modified immune therapy for cancer and allergy, targeting glycan binding receptors on skin resident antigens presenting cells that induce or inhibit immunity. Her other line of research is aimed to discover of glycan imposed regulatory immune imprinting by inflamed tissue and tumor microenvironment. She studies these questions in in-vivo mouse tumor models such as pancreatic cancer, lung and melanoma in patient derived tissues and human in-vitro models such as skin model, tumor tissues and complex 3D culture models to identify cellular communication at omics level in the context of tissue alterations.
She was awarded various NWO grants (PIONIER-ASPASIA), ERC Advanced-Eurostars and received the SPINOZA and van Loghem award for life time achievements in field of (Glyco)-Immunology, and is a member of the Royal Netherlands Academy of Sciences (KNAW).
Research Line
Glyco-code in cancer and inflammatory diseases
We explore the innate immune system, with a focus on dendritic cells and macrophages, to develop novel therapeutics in cancer and inflammatory diseases. Dendritic cells and macrophages have unique properties to act as sensors for local changes and respond to tissue injury by producing inflammatory mediators or help to eliminate injury and restore tissues. We study innate glycan binding receptors that recognize glycosylated structures on pathogens or (altered) tissues, modulate immune activating or regulatory programs of dendritic cells and myeloid cells. We investigate how binding of carbohydrates, altered due to tissue inflammation or wound healing processes, control modulation of immune cells through recognition/signaling of glycan binding receptors, such as C-type lectins and Siglecs. We explore how glycan- glycan binding receptor control the immune signature and regulate tumor growth or pathogen recognition and inflammation. Using transcriptomics and single cell sequencing we explore differential expression of glycosylation related genes in cancer patients tissues to unravel altered glycosylation signatures, to predict for survival benefit, response to therapy, and glycan induced immune modulation. We investigate how the glyco-code of the tumor-stromal network affects immune regulation. Using high dimensional cytometry and multiplex imaging we aim to define novel myeloid suppressive pathways in the tumor microenvironment of NSCLC, melanoma and PDAC. We also unravel how innate glycosylation in (tumor)tissue exert their effect on T cell and NK(T) cell function. New glycan immune regulatory programs will be studied in CRISPR-Cas9 generated glycan modified tumor/stromal mouse and human models, and glyco-code modifying therapies will be developed to revert suppression into tumor specific immunity. Next to cancer we study altered glycosylation processes in inflammatory diseases, such as rheumatoid arthritis, aimed to restore the immune balance towards suppression of the disease.

TME of the Lung cancer using multiplex imaging. Tumor (white), immune markers (colors)
Glycan modified immune therapy for cancer and allergy
Professional antigen-presenting cells such as dendritic cells (DC) and Langerhans cells (LC) have also the capability to induce long-term cellular immunity. We investigate glycan binding receptors such as C-type lectins and Siglecs on innate myeloid, macrophages and DC for targeting purposes to guide glycosylated antigens binding and uptake that influence antigen presentation, T cell differentiation and immune cell signaling. The cancer nano-vaccines we develop use specific glycans that target receptors such as DC-SIGN and Langerin that stimulate anti-tumor immunity, in human skin models and in-vivo mouse tumor models. Using high dimensional flow analysis local and systemic immune infiltrates and dynamics of the tumor microenvironment are studied. These DC targeting vaccines are combined with immune checkpoints or other combinatorial therapies that alter immune regulatory processes in the tumor-microenvironment. Immune inhibitory vaccines for treatment of auto-immune diseases and allergy use glycans that target glycans that interact with immune inhibitory receptors such as Siglecs. We explore T cell differentiation processes, antibody isotype responses, and the effect on innate immune cells, such as neutrophils and mast cells. Through fundamental discovery we aim to apply knowledge on glycan-DC modifying processes to be implemented in the treatment of cancer and inflammatory diseases.
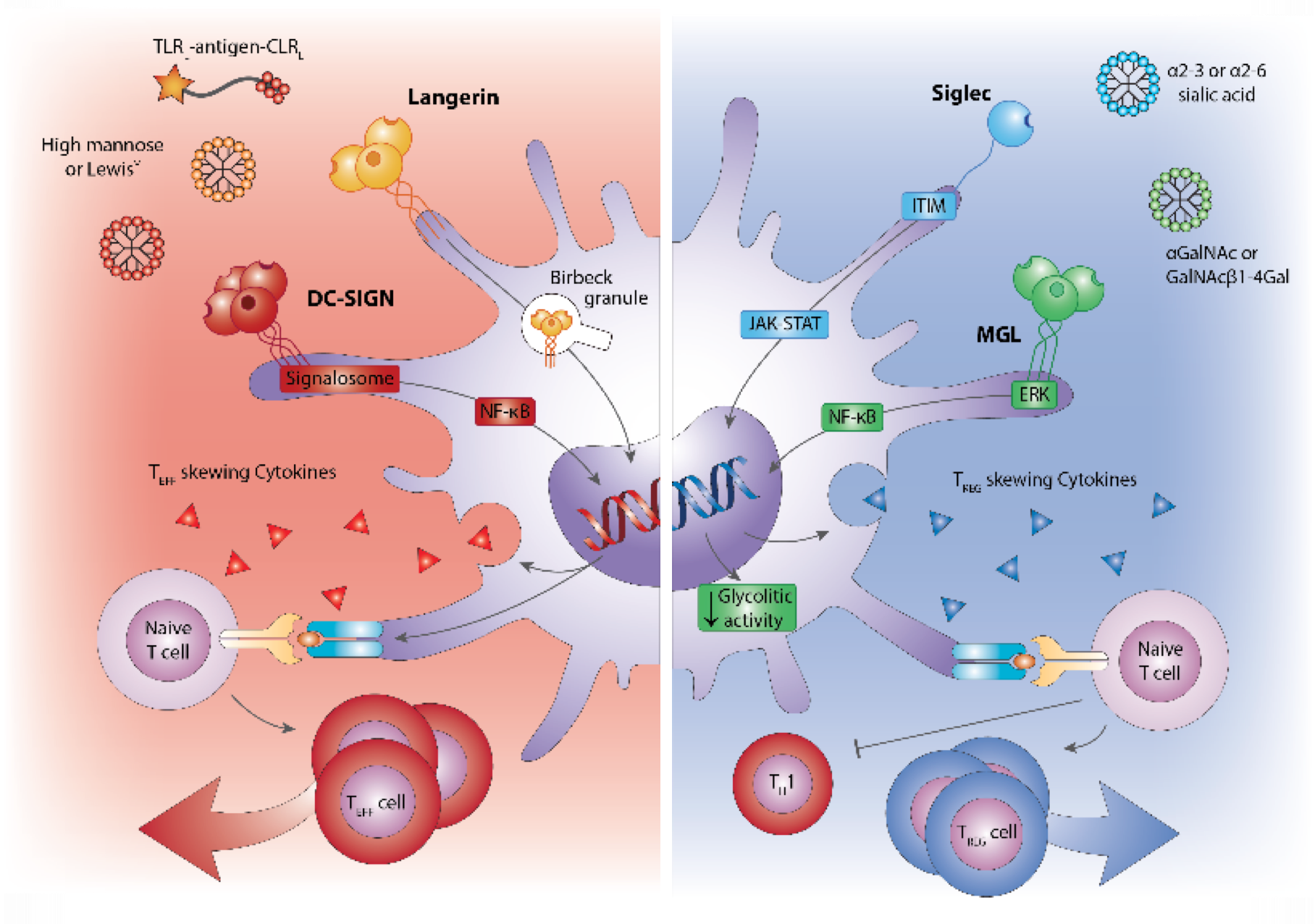
Glycan targeted vaccines (DC-SIGN/Langerin) for tumor immunity increasing T cell responses (red) or for reducing inflammatory responses, through Siglecs, aimed to induce tolerance (blue)
Key publications
- Glyco-Dendrimers as Intradermal Anti-Tumor Vaccine Targeting Multiple Skin DC Subsets. Duinkerken S, Horrevorts SK, Kalay H, Ambrosini M, Rutte L, de Gruijl TD, Garcia-Vallejo JJ, van Kooyk Y. Theranostics. 2019 Aug 12;9(20):5797-5809. doi: 10.7150/thno.35059
- The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018 Feb 5. doi: 10.1038/nri.2018.3.
- Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells.Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LA, Schetters ST, Engels S, Ambrosini M, Kalay H, Veninga H, den Haan JM, van Berkel LA, Samsom JN, Crocker PR, Sparwasser T, Berod L, Garcia-Vallejo JJ, van Kooyk Y, Unger WW. Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):3329-34.
- Glycan modification of antigen alters its intracellular routing in dendritic cells, promoting priming of T cells.Streng-Ouwehand I, Ho NI, Litjens M, Kalay H, Boks MA, Cornelissen LA, Kaur Singh S, Saeland E, Garcia-Vallejo JJ, Ossendorp FA, Unger WW, van Kooyk Y. Elife. 2016 Mar 21;5. pii: e11765. doi: 10.7554/eLife.11765.
- Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells Perdicchio M, Cornelissen LA, Streng-Ouwehand I, Engels S, Verstege MI, Boon L, Geerts D, Unger WW, van Kooyk Y. Oncotarget. 2016 Jan 5. doi: 10.18632/oncotarget.6822.
Group members

Aram de Haas, MSc
PhD Student
During my PhD within the DC4Balance consortium I am working on influencing the immune system by targeting specific C-type lectin receptors on antigen presenting cells with glycan-coated nanoparticles. The goal, depending on the formulation used, is to either induce an immune response against cancer, or dampen allergies. During my PhD I use various techniques such as multicolor (spectral) flow cytometry, moDC and T cell assays an in vivo models.

Brigitte-Carole Keumatio Doungtsop
PhD Student
The focus of my research is the induction of antigen-specific tolerance to house dust mite allergens through the use of allergens from house dust mite conjugated to sialic acid to target Siglecs on antigen presenting cells. I assess the capacity of these antigen presenting cells to induce T regulatory cells and suppress the induction T helper 2 cells. I also assess the capacity of these allergen-sialic acid conjugates to suppress the production of IgE from B cells and to suppress the activity of mast cells and eosinophils.

Chang Liu, MSc
PhD Student
The focus of my work is to search for some essential biomarkers in cancer-associated fibroblasts that could influence the metastasis of lung cancer. Furthermore, I want to reveal signalling pathways that those biomarkers relate to the metastasis and cell proliferation of NSCLC. For this project I make use of RNA sequencing data, CAM assay, IHC staining, Western Blotting and ELISA.

Dimitri Lindijer, MSc
PhD Student
My research is about understanding the tumor “glyco-code” in cancer for improvement of diagnosis, prediction and treatment. Aberrant glycosylation is a key feature during tumor development and has a role in every hallmark of cancer. Using both experimental and computational techniques, we analyze the expression of glycosylation-related genes and investigate the relationship between glycan expression and immune modulation.

Eleonora Nardini, MSc
PhD student
My project aims at exploring sialylation of plasma derived /recombinant FVIII with as a strategy to induce preventive or therapeutic immune tolerance in hemophilia A (HA) patients. The development of anti – FVIII antibodies in ~ 20% of patients presents a major threat for the success of replacement therapies. Therefore, targeting Siglecs on dendritic cells with α2,3 sialic acid – FVIII is an attractive option to reduce FVIII immunogenicity and ameliorate the current treatment for HA.

Eveline Li, PhD
Postdoctoral Researcher/Project manager (DC4U)
Project Lead at DC4U Technologies (spin-off company Yvette van Kooyk)
A lot of amazing and innovative research is perfomed in the group of Yvette van Kooyk. My main task is the management of all sialic acid-related research. We aim to translate the date into pre-clinical research packages which can be used for continued research in a clinical setting. I guide PhD students, manage the quality control, monitor the project progression, and ensure stakeholder satisfaction.

Fabrizio Chiodo, PhD
Visiting Fellow
I study carbohydrate-mediated host-pathogens interactions. Knowing the molecular basis of these interactions and the immunological cascades linked to these events, are key elements to 1. Desing and understand carbohydrate-based vaccines; 2. Understand the innate system exploring carbohydrates as "potentiators"; 3. Explore the effects of microbiota in the context of the tumor microenvironment (lung cancer for example)

Kelly Boelaars, MSc
PhD Student
I study stromal-immune cell interactions in the tumor microenvironment

Laura Kruijssen, MSc
Research technician
The focus of our research is finding immune therapies for cancer. On this moment the focus is on pancreatic cancer

Magali Coccimiglio, Biochemist
PhD student
I am the Early Stage Researcher 13 of the Glytunes network (https://www.glytunes.eu/). My project is focused on the study of sialylation, the process by which a glycan called sialic acid is added to proteins and lipids. An increased expression of sialic acid on the surface of cells is a hallmark of cancer, and this glycan can interact with receptors on immune cells called Siglecs. Consequently, the sialic acid-Siglec axis represents a novel immune checkpoint to be exploited for anti-cancer therapies. During my PhD, I will study this interaction and use different approaches to target it using both in vitro and in vivo models of melanoma, pancreatic cancer and lung cancer.

Sanne Duinkerken, PhD
Postdoctoral researcher
Teaching immunology in different bachelor and master courses, while continuing in the tumor-immunology research with emphasis on vaccination strategies.

Thomas van den Brekel, Ir.
PhD student
The focus of my research is the development of designer bacterial nanoparticles for cancer immunotherapy. We engineer the particles into semi systemic scaffolds that can be targeted for precise delivery and contain tailored immune modulatory properties. Subsequently, we analyze the distribution of the particles in vivo and test the prototype vaccines for therapeutic cancer treatment in vivo.
Other PI's
Sue Gibbs
Febe van Maldegem
Gijs Kooij
Elga de Vries
Sandra van Vliet
Reina Mebius
Jack van Horssen
Joke den Haan
Juan J. Garcia Vallejo
Marjolein van Egmond
Jack van Horssen

About
Jack van Horssen is associated professor in at the Department of Molecular Cell Biology & Immunology and visiting professor at the Biomed Institute in Hasselt, Belgium Throughout his scientific career he has been interested in understanding the pathogenetic basis of neurodegenerative disorders. His research line focused on the identification of pathogenic processes and molecular pathways underlying multiple sclerosis (MS) disease progression with a main focus on understanding the pathological key events that are involved in inflammation-driven neurodegeneration. In the last years his interests in teaching and the development of innovative teaching strategies has grown and he is currently responsible for the bachelor programme Medicine.
There are no internships at this moment.
Key publications
- A. Kamermans, M. Rijnsburger, A. Chakraborty, S. van der Pol, H.E. de Vries*, J. van Horssen*. Reduced Angiopoietin-Like 4 Expression in Multiple Sclerosis Lesions Facilitates Lipid Uptake by Phagocytes via Modulation of Lipoprotein-Lipase Activity. *Authors contribute equally. Front Immunol. 2019 May 3;10:950.
- C.E. Leurs, P. Podlesniy, R. Trullas, L. Balk, M.D. Steenwijk, A. Malekzadeh, F. Piehl, B.M. Uitdehaag, J. Killestein, J. van Horssen*, C.E. Teunissen*. Cerebrospinal fluid mtDNA concentration is elevated in multiple sclerosis disease and responds to treatment. Mult. Scler. 2018 4:472-480
- .G. Nijland, R.J. Molenaar, S.M. van der Pol, P. van der Valk, C.J. van Noorden, H.E. de Vries, J. van Horssen. Differential expression of glucose-metabolizing enzymes in multiple sclerosis lesions. Acta Neuropathol Commun. 2015, 1:79
- P.G. Nijland, M.E. Witte, B. van Het Hof, S. van der Pol, J. Bauer, H. Lassmann, P. van der Valk, H.E. de Vries, J. van Horssen. Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: implications for multiple sclerosis.Acta Neuropathol. Commun. 2014 1:170
- M.E. Witte, P.G. Nijland, J.A. Drexhage, W. Gerritsen, D. Geerts, B. van het Hof, A. Reijerkerk, H.E. de Vries, P. van der Valk, J. van Horssen. Reduced expression of PGC-1alpha partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol. 2013, 2:231-243
Group members

NAME, titles
JOB DESCRIPTION
RESEARCH DESCRIPTION
Other PI's
Sue Gibbs
Febe van Maldegem
Gijs Kooij
Elga de Vries
Sandra van Vliet
Reina Mebius
Yvette van Kooyk
Joke den Haan
Juan J. Garcia Vallejo
Marjolein van Egmond
Joke den Haan

About
Joke den Haan is associate professor in cellular immunology with a specific interest in the role of myeloid cells in adaptive immunity. She obtained her PhD from the University of Leiden in 1997 on the biochemical characterization of human minor histocompatibility antigens (cum laude) and did a post-doc at the University of Washington, Seattle, in the lab of Prof.dr. Mike J. Bevan on antigen cross-presentation by dendritic cell subsets (1998-2003). She joined the department of Molecular Cell Biology and Immunology in 2004 and her lab investigates the function of different types of macrophages and DCs subsets and develops cancer vaccines that specifically deliver antigens to these cell types to achieve optimal adaptive immune responses (please link to KWF movie). For these studies the group uses different types of in vitro, ex vivo, and in vivo model systems.
Research Line
Function of CD169-expressing macrophages and dendritic cells
CD169+ macrophages are localized at the marginal zone of the spleen and the subcapsular sinus of lymph nodes where they capture pathogens and extracellular vesicles. We have previously shown that antigens that are bound by splenic CD169+ macrophages are presented to B cells and transferred to cross-presenting dendritic cells that activate CD8+ and CD4+ T cell responses. We investigate the interactions of CD169+ macrophages with dendritic cells and other immune cells.
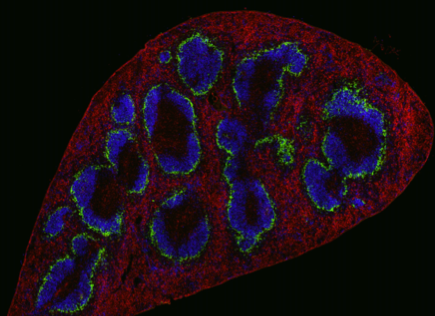
Recently, a CD169-expressing Axl+ dendritic cell subset was discovered in human PBMC. We can detect this cell type in healthy individuals and cancer patients and discovered that liposomal nanovaccines that bind to CD169 on these cells are taken up and presented to T cells.

Development of cancer nanovaccines
We have generated different types of nanovaccines that target cancer antigens and adjuvant specifically to CD169+ macrophages and dendritic cells in mice and men. For this we utilize CD169-specific antibodies that are conjugated to cancer antigens in the form of proteins or peptides. In addition, we have generated liposomes that contain physiological ligands for CD169 to target cancer antigens and adjuvant to CD169+ macrophages and dendritic cells. Current studies are focused on the type of adjuvant and antigen to be included for optimal immune responses. In addition, we are generating nanobodies for human CD169 and will include them in different types of nanovaccins.
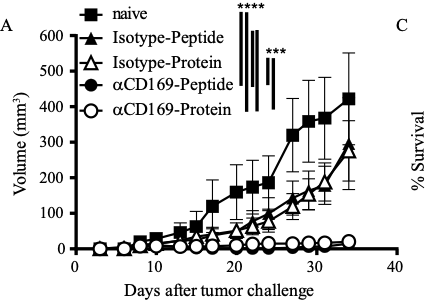
Key publications
- Affandi, A.J., Grabowska, J., Olesek, K., Lopez Venegas M., Barbaria, A., Rodríguez, E., Mulder, P.P.G., Pijffers, H.J., Ambrosini, M., Kalay, H., O’Toole, T., Zwart, E.S., Kazemier, G., Nazmi, K., Bikker, F.J., Stöckl, J., van den Eertwegh, A.J.M., de Gruijl, T.D., Storm, G., van Kooyk, Y., den Haan, J.M.M. 2020.
Selective tumor antigen vaccine delivery to human CD169+ antigen presenting cells using ganglioside-liposomes. PNAS accepted. We show in this paper that liposomes that contain gangliosides can bind to human CD169+ dendritic cells and are presented to T cells. - van Dinther, D., H. Veninga, S. Iborra, E. G. F. Borg, L. Hoogterp, K. Olesek, M. R. Beijer, S. T. T. Schetters, H. Kalay, J. J. Garcia-Vallejo, K. L. Franken, L. B. Cham, K. S. Lang, Y. van Kooyk, D. Sancho, P. R. Crocker, and J. M. M. den Haan. 2018.
Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8+ T Cell Cross-Priming. Cell Rep 22: 1484-1495. In this paper we describe that CD169 enables adhesion and antigen transfer to cross-presenting dendritic cells and that DNGR-1 mediated activation stimulates cross-presentation. PMID: 29425504 - Veninga, H., E. G. Borg, K. Vreeman, P. R. Taylor, H. Kalay, K. Y. van, G. Kraal, L. Martinez-Pomares, and J. M. den Haan. 2015.
Antigen targeting reveals splenic CD169 macrophages as promoters of germinal center B-cell responses.
Eur. J. Immunol 45: 747-757. In this paper we show that antigen targeting to CD169+macrophages stimulates strong germinal center B cell responses and that CD169+ macrophages retain intact antigen on their surface for two days. PMID: 25487358 - Backer, R., T. Schwandt, M. Greuter, M. Oosting, F. Jungerkes, T. Tuting, L. Boon, T. O’Toole, G. Kraal, A. Limmer, and J. M. den Haan. 2010.
Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells.
Proc. Natl. Acad. Sci. U. S. A 107: 216-221. This is our first paper showing that antigens are transferred from CD169+macrophages to cross-presentation DCs for CD8+ T cell activation. PMID: 20018690 - den Haan, J. M., S. M. Lehar, and M. J. Bevan.
CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med 192: 1685-1696. This paper is the first demonstration that CD8+DCs (currently named cDC1) selectively cross-present cell-associated antigens. PMID: 11120766
Group members

Alsya Affandi, PhD
Postdoctoral researcher
My research focuses on developing nanovaccines targeting antigen-presenting-cells such as dendritic cells and macrophages, to stimulate T cell responses against cancer or pathogens. A variety of nanovaccine platforms are used including liposome and single-domain antibodies. For my research, I use spectral/imaging flow cytometry, immunoassays, microscopy, and analysis of single-cell RNA sequencing datasets.

Aru Zeling Wang, MSc
PhD student
My project is to develop a versatile cancer vaccine format in which patient-specific tumor antigens can be conjugated to antibodies specific for dendritic cells to stimulate immune responses.

Joanna Grabowska, MSc
PhD student
In my project I use CD169 targeting liposomes to efficiently deliver the antigen to splenic CD169+ macrophages. Apart from the quantitative and qualitative characterization of liposome-induced adaptive immune responses I also try to identify key mechanisms underlying these immune responses by investigating the contribution of cDC1, CD169+ macrophages and platelets. I perform in vivo immunizations and collect flow cytometry data, but also ELISA-based data and in vitro data.

Maarten Nijen Twilhaar, MSc
PhD student
I work on liposomal cancer vaccines that target to CD169-expressing macrophages, a specific subset of macrophages in the spleen. For this, we surface decorate liposomes with targeting moieties and include various antigens and adjuvants in our liposomes. Using various in vitro assays, in vivo models and multicolor flow cytometry readouts, we aim to augment the efficacy of liposomal cancer vaccination.

Rianne Bouma, MSc
PhD student
My project focuses on creating a vaccine using novel molecules targeted to CD169+ macrophages. These molecules will be conjugated to liposomes which have cancer antigen encapsulated. This will elicit a macrophage/dendritic cell mediated immune response against the specific tumor.
Other PI's
Sue Gibbs
Febe van Maldegem
Gijs Kooij
Elga de Vries
Sandra van Vliet
Reina Mebius
Yvette van Kooyk
Jack van Horssen
Juan J. Garcia Vallejo
Marjolein van Egmond
Juan J. Garcia Vallejo

About
Juan J. Garcia Vallejo is Associate Professor and Principal Investigator at the Department of Molecular Cell Biology & Immunology. Juan has always been interested in combining his medical background with a keen interest in technology-driven biomedical research. He leads the Immune System CytOMICs research group, where he applies cutting-edge cytometry and computational methods for the characterization of the interplay between different components of the immune system and the identification of immune-based biomarkers in different disease models, including brain cancer, COVID-19, and exposure to microplastics. Juan completed an MBA in 2017 with a thesis on the management of core facilities in an academic research environment. Since 2016, he acts as Scientific Director of the Microscopy and Cytometry Core Facility of the Amsterdam UMC – Location VUmc. Juan is an active member of the International Society for the Advancement of Cytometry (ISAC) and the Core Technologies for Life Sciences (CTLS) Society.
Research Line
Characterization of the human immune system cytome (theme Tumor Immunology)
Two of the most relevant characteristics of the immune system are its highly complex heterogeneity in terms of cell types and functional states, and, the interconnectedness of all these cell subsets. Both properties are critical for the functioning of the immune system in protecting us from infections while maintaining homeostasis; but, at the same time, make this organ extremely difficult to characterize in detail. With the advent of highly multiplexed single-cell technologies we are getting closer to characterize the immune system cytome (the collection of all different cell subsets and cell states within an organ, including their interrelations) which will be critical for the identification of correlates of disease or immunological interventions as well as for elucidating the underlying mechanisms of immunity.
Two major technical advances in the field of cytometry in recent years have made possible the simultaneous characterization of more than 40 markers at a single cell level. Both mass cytometry (cytometry by time-of-flight or CyTOF) and spectral flow cytometry are allowing the routine implementation of comprehensive immunophenotyping panels in research and clinical environments. Despite of the advances, the currently available chemistries present a number of limitations. In CyTOF, the loading of metal isotopes cannot be controlled and only a few elements can be successfully loaded. In spectral flow cytometry, new fluorochromes are needed to fully exploit the configuration of current instruments. In this project, we are exploring the development of new polymer chemistries and computational methods to extend mass and spectral flow cytometry beyond the current limits of the technology and bring it to the level of targeted proteomics at the single cell level.
A functional immune atlas of glioblastoma (theme Tumor Immunology)
Glioblastomas are the most aggressive brain malignancies, for which immunotherapy has so far failed to prolong survival. Glioblastoma-associated immune infiltrates are dominated by tumor-associated macrophages and microglia, which are key mediators of immune suppression and resistance to immunotherapy. We have recently demonstrated that tumor-intrinsic mechanisms, such as altered glycosylation, have an impact in the distribution and phenotype of tumor-infiltrating myeloid cells. Interestingly, altered tumor glycosylation also had a distinct impact on the peripheral immune compartment, even though it is well known that glioblastomas do not metastasize. Our goal is to understand the heterogeneity of the immune infiltrate in glioblastoma with the ultimate aim to identify potential therapeutic targets. In addition, we also focus in the study of the peripheral immune system to explore the presence of signatures that can predict immune composition in the tumor and/or can have predictive/prognostic value.
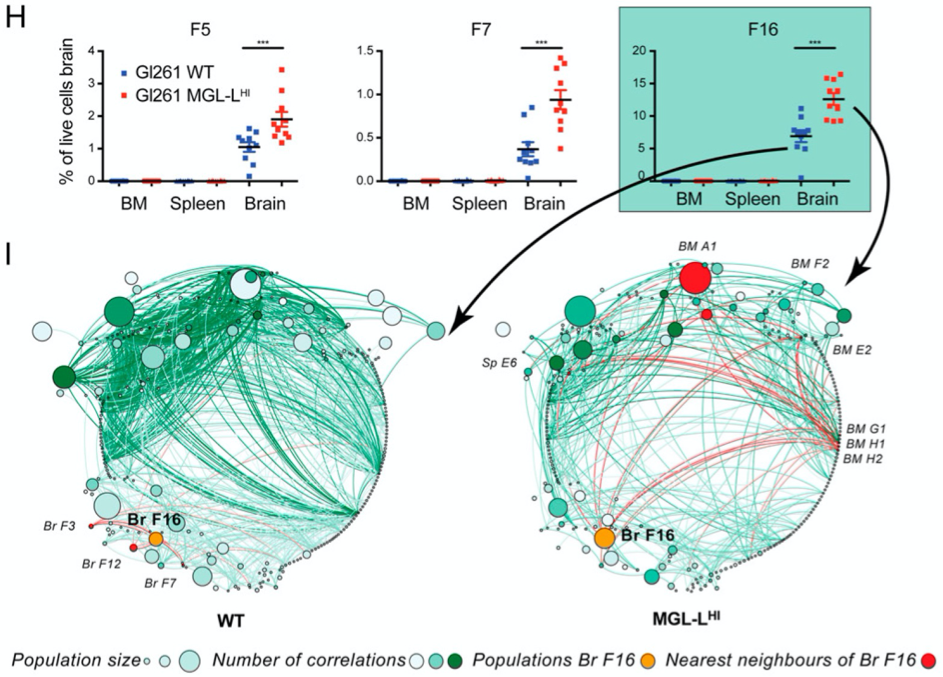
Emerging research lines in the group
COVID-19 immune monitoring: The pandemic Coronavirus-disease 19 (COVID-19), caused by infection with the SARS-CoV-2 virus is characterized by a heterogeneous clinical course. While the majority of patients experience only mild symptoms, a substantial proportion develops severe disease with increasing hypoxia up to acute respiratory distress syndrome (ARDS), a disease manifestation that seems to be strongly linked to a Cytokine Release Syndrome (CRS). Importantly, severe course of COVID-19 cannot be explained only by patients’ age and concurrent diseases, because young and otherwise healthy subjects can develop lethal complications of COVID-19, suggesting that unknown susceptibility factors are involved. There is an urgent need to develop better diagnostic/prognostic tools for CRS in COVID-19 as early treatment is expected to either prevent or alleviate the severity of the ARDS. This is of special relevance while there is no readily available vaccine and, yet, more cases of COVID-19 patients are expected in successive pandemic waves of SARS-CoV-2 or, potentially, other corona viruses. In collaboration with Dr. F. van Wijk (UMCU) and Prof. S. Nezhentsev (Amsterdam UMC), we propose to use genetics and broad immunophenotyping by spectral flow cytometry to (a) characterize the immune response of COVID-19 patients at risk of developing CRS and (b) identify susceptibility factors for the development of CRS in the context of SARS-CoV-2 infection.
Microplastics and health: Our health is intrinsically related to the quality of the environment. Humans are exposed to unknown quantities of plastic particulate debris on a daily basis via air water and food. That’s why it is important to determine what adverse health outcomes may arise out of plastic particle pollution. One of the important mechanisms we think is involved in the toxicity of these particles is an interference with homeostatic immune function. This study seeks to answer the question if human blood actually contains PET used in textiles and packaging or Teflon powders extensively used in lubricants, inks and thermoplastics. And if so, what kind of hazardous immune system effects can we expect? In Collaboration with Dr. H. Leslie (VU), we are using cutting edge analytical method for the determination of microplastics in blood and, using a human blood in vitro exposure model, we seek to understand the immunological signals that show us how small plastic particles may be interfering with homeostatic immune function.
Key publications
- Crommentuijn MHW, Schetters STT, Dusoswa SA, Kruijssen LJW, Garcia-Vallejo JJ, van Kooyk Y. Immune involvement of the contralateral hemisphere in a glioblastoma mouse model. J Immunother Cancer. 2020; 8 (1): e000323.
- Dusoswa SA, Verhoeff J, (…), Garcia-Vallejo JJ. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc Natl Acad Sci U S A. 2020; 117 (7): 3693-3703.
- Dusoswa SA, (…), Garcia-Vallejo JJ. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J Extracell Vesicles. 2019; 8 (1): 1648995.
- Dusoswa SA, Verhoeff J, Garcia-Vallejo JJ. OMIP-054: Broad Immune Phenotyping of Innate and Adaptive Leukocytes in the Brain, Spleen, and Bone Marrow of an Orthotopic Murine Glioblastoma Model by Mass Cytometry. Cytometry A. 2019; 95 (4): 422-426.
- Duinkerken S, van Kooyk Y, Garcia-Vallejo JJ. Human cytomegalovirus-based immunotherapy to treat glioblastoma: Into the future. Oncoimmunology. 2016; 5 (9): e1214791.
Group members

Maartje Rietdijk, MSc
PhD student
My project is focused on understanding the exposure and immunotoxicity of micro- and nanoplastic (MNP) contaminants in humans by using in vitro models.

Xiangming Cai, MSc
PhD student
My work focuses on the immune monitoring of glioblastomas.