
About
Yvette van Kooyk is professor in Molecular Cell Biology and Immunology, head of the department Molecular Cell Biology and Immunology and PI of the Dendritic Cell Immunobiology group. Van Kooyk’s research team studies innate and adaptive immune responses guided by glycosylation. Her team unravels cellular communication driven by modified glycoproteins/lipids in cancer, allergy and autoimmunity. Central in her work is the development of new glycan modified immune therapy for cancer and allergy, targeting glycan binding receptors on skin resident antigens presenting cells that induce or inhibit immunity. Her other line of research is aimed to discover of glycan imposed regulatory immune imprinting by inflamed tissue and tumor microenvironment. She studies these questions in in-vivo mouse tumor models such as pancreatic cancer, lung and melanoma in patient derived tissues and human in-vitro models such as skin model, tumor tissues and complex 3D culture models to identify cellular communication at omics level in the context of tissue alterations.
She was awarded various NWO grants (PIONIER-ASPASIA), ERC Advanced-Eurostars and received the SPINOZA and van Loghem award for life time achievements in field of (Glyco)-Immunology, and is a member of the Royal Netherlands Academy of Sciences (KNAW).
Research Line
Glyco-code in cancer and inflammatory diseases
We explore the innate immune system, with a focus on dendritic cells and macrophages, to develop novel therapeutics in cancer and inflammatory diseases. Dendritic cells and macrophages have unique properties to act as sensors for local changes and respond to tissue injury by producing inflammatory mediators or help to eliminate injury and restore tissues. We study innate glycan binding receptors that recognize glycosylated structures on pathogens or (altered) tissues, modulate immune activating or regulatory programs of dendritic cells and myeloid cells. We investigate how binding of carbohydrates, altered due to tissue inflammation or wound healing processes, control modulation of immune cells through recognition/signaling of glycan binding receptors, such as C-type lectins and Siglecs. We explore how glycan- glycan binding receptor control the immune signature and regulate tumor growth or pathogen recognition and inflammation. Using transcriptomics and single cell sequencing we explore differential expression of glycosylation related genes in cancer patients tissues to unravel altered glycosylation signatures, to predict for survival benefit, response to therapy, and glycan induced immune modulation. We investigate how the glyco-code of the tumor-stromal network affects immune regulation. Using high dimensional cytometry and multiplex imaging we aim to define novel myeloid suppressive pathways in the tumor microenvironment of NSCLC, melanoma and PDAC. We also unravel how innate glycosylation in (tumor)tissue exert their effect on T cell and NK(T) cell function. New glycan immune regulatory programs will be studied in CRISPR-Cas9 generated glycan modified tumor/stromal mouse and human models, and glyco-code modifying therapies will be developed to revert suppression into tumor specific immunity. Next to cancer we study altered glycosylation processes in inflammatory diseases, such as rheumatoid arthritis, aimed to restore the immune balance towards suppression of the disease.

TME of the Lung cancer using multiplex imaging. Tumor (white), immune markers (colors)
Glycan modified immune therapy for cancer and allergy
Professional antigen-presenting cells such as dendritic cells (DC) and Langerhans cells (LC) have also the capability to induce long-term cellular immunity. We investigate glycan binding receptors such as C-type lectins and Siglecs on innate myeloid, macrophages and DC for targeting purposes to guide glycosylated antigens binding and uptake that influence antigen presentation, T cell differentiation and immune cell signaling. The cancer nano-vaccines we develop use specific glycans that target receptors such as DC-SIGN and Langerin that stimulate anti-tumor immunity, in human skin models and in-vivo mouse tumor models. Using high dimensional flow analysis local and systemic immune infiltrates and dynamics of the tumor microenvironment are studied. These DC targeting vaccines are combined with immune checkpoints or other combinatorial therapies that alter immune regulatory processes in the tumor-microenvironment. Immune inhibitory vaccines for treatment of auto-immune diseases and allergy use glycans that target glycans that interact with immune inhibitory receptors such as Siglecs. We explore T cell differentiation processes, antibody isotype responses, and the effect on innate immune cells, such as neutrophils and mast cells. Through fundamental discovery we aim to apply knowledge on glycan-DC modifying processes to be implemented in the treatment of cancer and inflammatory diseases.
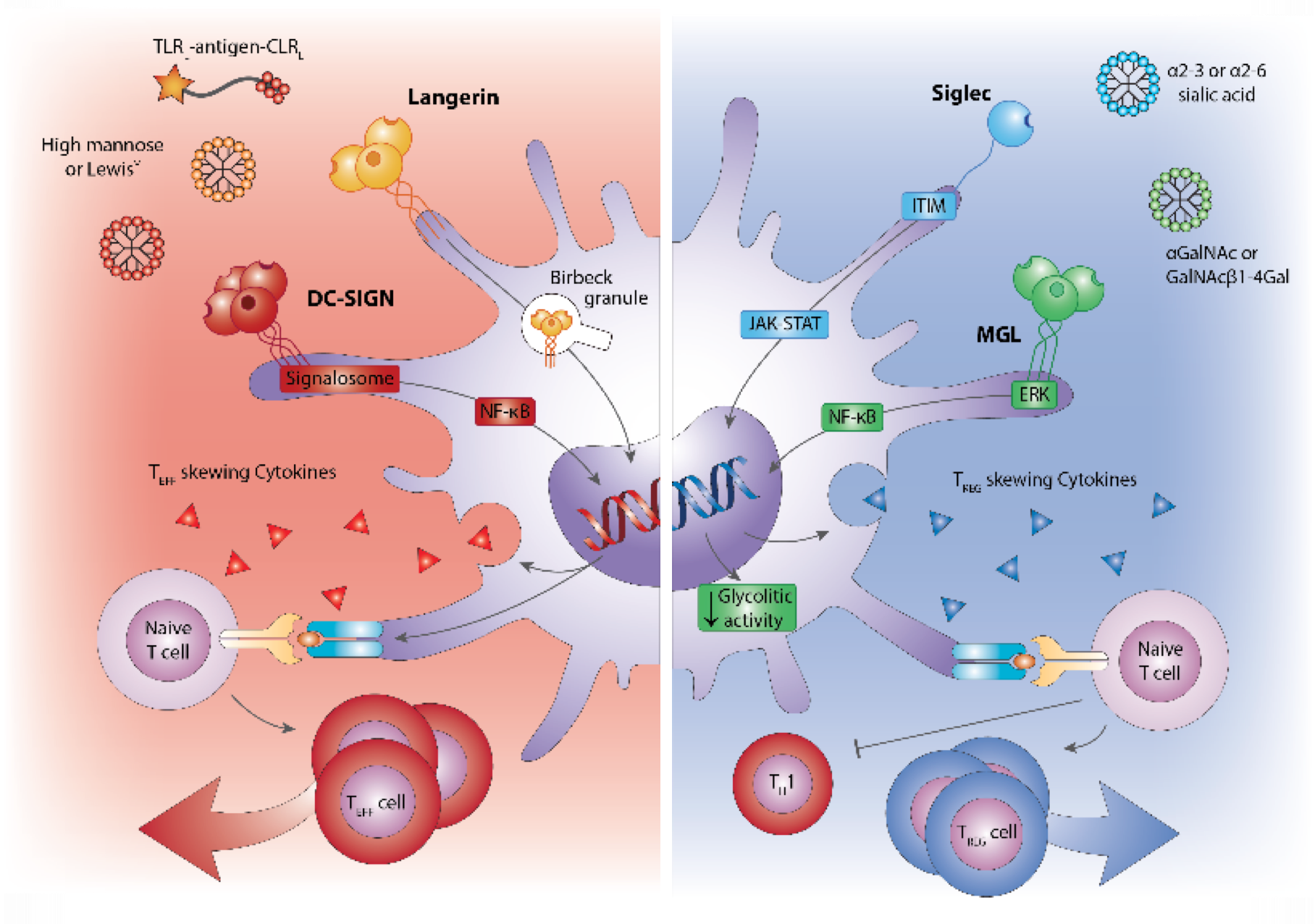
Glycan targeted vaccines (DC-SIGN/Langerin) for tumor immunity increasing T cell responses (red) or for reducing inflammatory responses, through Siglecs, aimed to induce tolerance (blue)
Key publications
- Glyco-Dendrimers as Intradermal Anti-Tumor Vaccine Targeting Multiple Skin DC Subsets. Duinkerken S, Horrevorts SK, Kalay H, Ambrosini M, Rutte L, de Gruijl TD, Garcia-Vallejo JJ, van Kooyk Y. Theranostics. 2019 Aug 12;9(20):5797-5809. doi: 10.7150/thno.35059
- The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018 Feb 5. doi: 10.1038/nri.2018.3.
- Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells.Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LA, Schetters ST, Engels S, Ambrosini M, Kalay H, Veninga H, den Haan JM, van Berkel LA, Samsom JN, Crocker PR, Sparwasser T, Berod L, Garcia-Vallejo JJ, van Kooyk Y, Unger WW. Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):3329-34.
- Glycan modification of antigen alters its intracellular routing in dendritic cells, promoting priming of T cells.Streng-Ouwehand I, Ho NI, Litjens M, Kalay H, Boks MA, Cornelissen LA, Kaur Singh S, Saeland E, Garcia-Vallejo JJ, Ossendorp FA, Unger WW, van Kooyk Y. Elife. 2016 Mar 21;5. pii: e11765. doi: 10.7554/eLife.11765.
- Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells Perdicchio M, Cornelissen LA, Streng-Ouwehand I, Engels S, Verstege MI, Boon L, Geerts D, Unger WW, van Kooyk Y. Oncotarget. 2016 Jan 5. doi: 10.18632/oncotarget.6822.
Group members

Aram de Haas, MSc
PhD Student
During my PhD within the DC4Balance consortium I am working on influencing the immune system by targeting specific C-type lectin receptors on antigen presenting cells with glycan-coated nanoparticles. The goal, depending on the formulation used, is to either induce an immune response against cancer, or dampen allergies. During my PhD I use various techniques such as multicolor (spectral) flow cytometry, moDC and T cell assays an in vivo models.

Brigitte-Carole Keumatio Doungtsop
PhD Student
The focus of my research is the induction of antigen-specific tolerance to house dust mite allergens through the use of allergens from house dust mite conjugated to sialic acid to target Siglecs on antigen presenting cells. I assess the capacity of these antigen presenting cells to induce T regulatory cells and suppress the induction T helper 2 cells. I also assess the capacity of these allergen-sialic acid conjugates to suppress the production of IgE from B cells and to suppress the activity of mast cells and eosinophils.

Chang Liu, MSc
PhD Student
The focus of my work is to search for some essential biomarkers in cancer-associated fibroblasts that could influence the metastasis of lung cancer. Furthermore, I want to reveal signalling pathways that those biomarkers relate to the metastasis and cell proliferation of NSCLC. For this project I make use of RNA sequencing data, CAM assay, IHC staining, Western Blotting and ELISA.

Dimitri Lindijer, MSc
PhD Student
My research is about understanding the tumor “glyco-code” in cancer for improvement of diagnosis, prediction and treatment. Aberrant glycosylation is a key feature during tumor development and has a role in every hallmark of cancer. Using both experimental and computational techniques, we analyze the expression of glycosylation-related genes and investigate the relationship between glycan expression and immune modulation.

Eleonora Nardini, MSc
PhD student
My project aims at exploring sialylation of plasma derived /recombinant FVIII with as a strategy to induce preventive or therapeutic immune tolerance in hemophilia A (HA) patients. The development of anti – FVIII antibodies in ~ 20% of patients presents a major threat for the success of replacement therapies. Therefore, targeting Siglecs on dendritic cells with α2,3 sialic acid – FVIII is an attractive option to reduce FVIII immunogenicity and ameliorate the current treatment for HA.

Eveline Li, PhD
Postdoctoral Researcher/Project manager (DC4U)
Project Lead at DC4U Technologies (spin-off company Yvette van Kooyk)
A lot of amazing and innovative research is perfomed in the group of Yvette van Kooyk. My main task is the management of all sialic acid-related research. We aim to translate the date into pre-clinical research packages which can be used for continued research in a clinical setting. I guide PhD students, manage the quality control, monitor the project progression, and ensure stakeholder satisfaction.

Fabrizio Chiodo, PhD
Visiting Fellow
I study carbohydrate-mediated host-pathogens interactions. Knowing the molecular basis of these interactions and the immunological cascades linked to these events, are key elements to 1. Desing and understand carbohydrate-based vaccines; 2. Understand the innate system exploring carbohydrates as "potentiators"; 3. Explore the effects of microbiota in the context of the tumor microenvironment (lung cancer for example)

Kelly Boelaars, MSc
PhD Student
I study stromal-immune cell interactions in the tumor microenvironment

Laura Kruijssen, MSc
Research technician
The focus of our research is finding immune therapies for cancer. On this moment the focus is on pancreatic cancer

Magali Coccimiglio, Biochemist
PhD student
I am the Early Stage Researcher 13 of the Glytunes network (https://www.glytunes.eu/). My project is focused on the study of sialylation, the process by which a glycan called sialic acid is added to proteins and lipids. An increased expression of sialic acid on the surface of cells is a hallmark of cancer, and this glycan can interact with receptors on immune cells called Siglecs. Consequently, the sialic acid-Siglec axis represents a novel immune checkpoint to be exploited for anti-cancer therapies. During my PhD, I will study this interaction and use different approaches to target it using both in vitro and in vivo models of melanoma, pancreatic cancer and lung cancer.

Sanne Duinkerken, PhD
Postdoctoral researcher
Teaching immunology in different bachelor and master courses, while continuing in the tumor-immunology research with emphasis on vaccination strategies.

Thomas van den Brekel, Ir.
PhD student
The focus of my research is the development of designer bacterial nanoparticles for cancer immunotherapy. We engineer the particles into semi systemic scaffolds that can be targeted for precise delivery and contain tailored immune modulatory properties. Subsequently, we analyze the distribution of the particles in vivo and test the prototype vaccines for therapeutic cancer treatment in vivo.










