
About
Jan Van den Bossche is Associate Professor at the Department of Molecular Cell Biology and Immunology at Amsterdam UMC. He leads the Translational Macrophage Immunometabolism group IMMUNOMETLAB. His young and enthusiastic team specializes in the immune/metabolic profiling and targeting of macrophages and other immune cells, particularly in the context of cancer and cardiometabolic diseas
Research Line
Our research aims to explain how metabolic reprogramming regulates macrophage subsets in different settings, focusing on cancer and cardiovascular disease. By unravelling key questions in macrophage immunometabolism, our overall goal is to demonstrate whether and how targeting macrophage metabolism could be used for future therapy.
Our distinct research lines investigate how metabolic enzymes like ATP Citrate Lyase and (immuno)metabolites like succinate and itaconate control macrophage responses and disease progression.
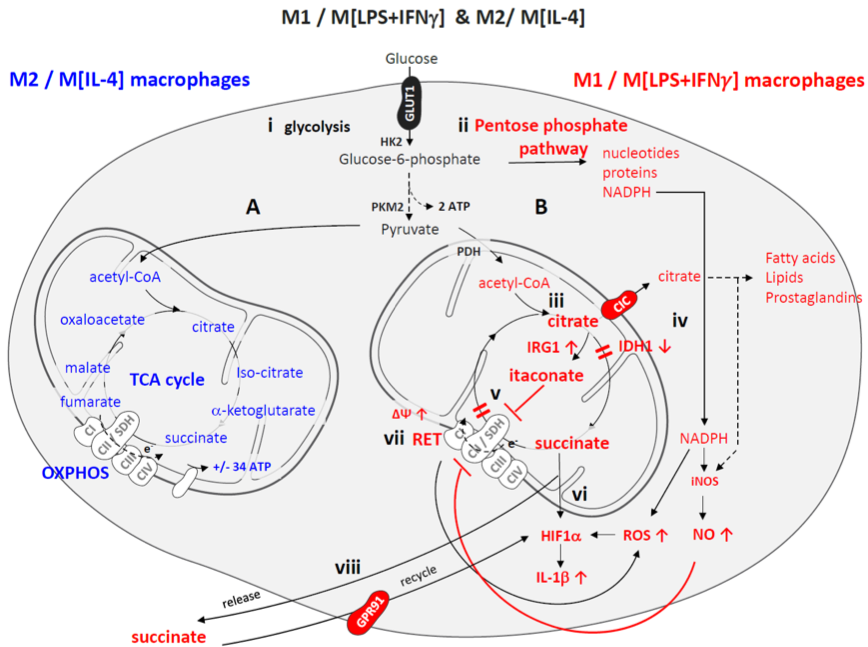
Metabolic rewiring in macrophage subsets
(source: Van den Bossche, O’Neill & Menon, Trends in Immunology, 2017)
Our distinct research lines investigate how metabolic enzymes like ATP Citrate Lyase and (immuno)metabolites like succinate and itaconate control macrophage responses and disease progression.
Key publications
-
Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques
Baardman, J., Verberk, S. G. S., van der Velden, S., Gijbels, M. J. J., van Roomen, C. P. P. A., Sluimer, J. C., Broos, J. Y., Griffith, G. R., Prange, K. H. M., van Weeghel, M., Lakbir, S., Molenaar, D., Meinster, E., Neele, A. E., Kooij, G., de Vries, H. E., Lutgens, E., Wellen, K. E., de Winther, M. P. J. & Van den Bossche, J., 1 Dec 2020, In: Nature communications. 11, 1, 6296. -
Immunometabolism in the Single-Cell Era
Artyomov, M. N. & Van den Bossche, J., 3 Nov 2020, In: Cell metabolism. 32, 5, p. 710-725 16 p. - Single-cell metabolic profiling of human cytotoxic T cells
Hartmann, F. J., Mrdjen, D., McCaffrey, E., Glass, D. R., Greenwald, N. F., Bharadwaj, A., Khair, Z., Verberk, S. G. S., Baranski, A., Baskar, R., Graf, W., Van Valen, D., Van den Bossche, J., Angelo, M. & Bendall, S. C., Feb 2021, In: Nature biotechnology. 39, 2, p. 186-197 12 p.
Team

Translational Macrophage Immunometabolism group “ImmunoMetLab” – Kyra de Goede, Sanne Verberk, Jan Van den Bossche, Elisa Meinster, Karl Harber (left to right, august 2019)
Sanne Verberk’s PhD aims to target immunometabolic circuits in atherosclerotic macrophages to improve their function and disease outcome. This work is funded by a Netherlands Heart Foundation senior researcher grant.
Kyra de Goede is funded by a CCA (Cancer Center Amsterdam) PhD grant and studies macrophage immunometabolism and immunometabolites in the tumor microenvironment.
Karl Harber is appointed on a ACS (Amsterdam Cardiovascular Sciences) PhD grant and investigates immunometabolites in the context of atherosclerosis in collaboration with Prof. Menno de Winther and Michel van Weeghel at AMC.
Elisa Meinster is research technician in our group and supports distinct research lines investigating how metabolic alterations in macrophages drive their function and disease outcome. Elisa is funded by a CCA Proof-of-concept grant and an European ERA-CVD consortium grant.










